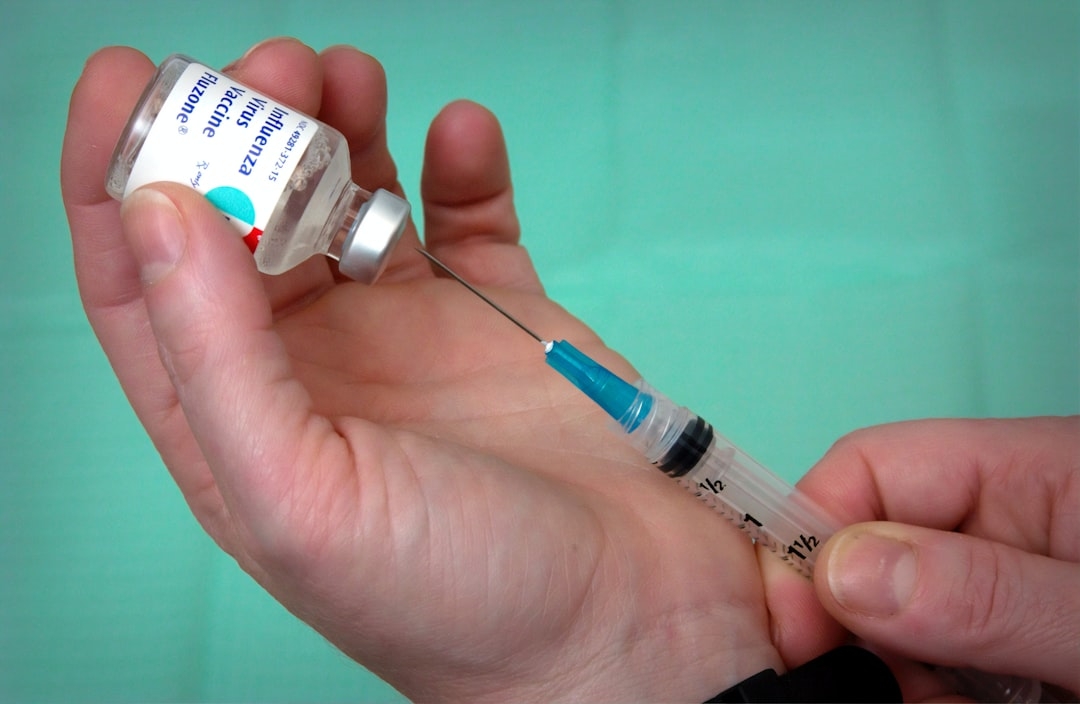
You've probably heard the familiar advice: get your flu shot every year. It's a cornerstone of public health, a seemingly simple solution to an annual scourge. But what if I told you that the current approach, while effective to a degree, is like patching a leaky dam? The flu virus is a master of disguise, constantly evolving, making vaccine efficacy a moving target. We’ve all experienced the frustration of the 'flu that got through,' or the nagging question of whether this year's vaccine will truly protect us. It turns out, the very fundamental way influenza operates might hold the key to its ultimate downfall, and the tool for this revolution could be CRISPR gene editing.
What It Really Is: The Unseen Battleground Within Your Cells
Influenza isn't just an external invader; it's a microscopic saboteur that hijacks your own cellular machinery. When the flu virus enters your body, it’s not content with simply infecting a few cells. Its primary goal is to replicate, and to do that, it needs to commandeer the host cell’s resources. A critical part of this process is the virus’s RNA (ribonucleic acid), which carries the genetic instructions for making more viruses. Influenza's RNA is segmented, meaning it's broken into several pieces. This segmentation is both a strength and a potential weakness. It allows the virus to easily reassort its genetic material with other flu viruses, a process called antigenic drift and shift, which is why we need new vaccines annually. However, targeting the fundamental machinery that handles this RNA replication is where innovative research is focusing its efforts.
How It Actually Works: CRISPR as a Molecular Scalpel for the Flu
This is where CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) enters the picture. You've likely heard of CRISPR in the context of human gene editing, but its precision and programmability make it an exceptional tool for targeting viral genetic material. Think of CRISPR as a highly sophisticated molecular GPS system combined with a pair of molecular scissors. It consists of two main components: a guide RNA (gRNA) that acts as the GPS, directing the system to a specific sequence of genetic material, and an enzyme, typically Cas9, that acts as the scissors, cutting the DNA or RNA at that precise location. In the fight against influenza, researchers are designing guide RNAs to specifically target essential segments of the flu virus's RNA. When the CRISPR-Cas9 system is introduced into infected cells, the gRNA guides Cas9 to the viral RNA. Cas9 then cleaves, or cuts, the viral RNA, effectively disabling it. This prevents the virus from replicating and creating new progeny. It’s a direct, targeted attack on the virus’s ability to reproduce, rather than an indirect approach that relies on boosting the host's immune system.
The Elegance of Targeting Viral Replication
The beauty of this approach lies in its specificity. Unlike broad-spectrum antivirals that can have off-target effects, CRISPR can be programmed to target only the viral RNA. The flu virus has specific RNA segments that are crucial for its replication cycle. By cutting these segments, the virus is effectively disarmed before it can even begin to make copies of itself. This is a fundamental departure from current flu treatments, which primarily focus on inhibiting viral entry or release from cells. Targeting replication directly offers a more robust way to halt the infection at its source. Researchers are exploring various strategies, including delivering CRISPR components to cells via nanoparticles or viral vectors, aiming for efficient delivery to infected tissues.
Common Misconceptions: It's Not About Editing *Your* Genes
One of the biggest hurdles in public understanding of CRISPR is the confusion with human gene editing. When people hear 'CRISPR,' they often think of permanent changes to our own DNA, with all the ethical and safety concerns that entails. It's crucial to understand that in this application, CRISPR is being used as an antiviral agent. The target is the viral RNA, not the host's DNA. The CRISPR components are transient; they are designed to act within the infected cell for a limited time to disable the virus. Once the viral replication is stopped and the viral RNA is cleared, the CRISPR machinery is also naturally degraded. This is fundamentally different from editing the human genome, which would involve making changes to your permanent genetic code. The goal here is to fight the virus, not to alter your biology.
Advanced Use Cases: Beyond Seasonal Flu
While the immediate application is to combat seasonal influenza, the principles behind this CRISPR-based approach could extend to other RNA viruses. Imagine a future where a single, programmable CRISPR system could be rapidly adapted to target novel emerging viruses, much faster than traditional vaccine development. The segmented nature of influenza's genome also presents an opportunity for developing broad-spectrum antiviral strategies. By targeting highly conserved regions across different influenza strains, it might be possible to create a single treatment effective against a wider range of flu viruses, including those that cause pandemics. This would represent a monumental leap in our preparedness for future global health crises.
A Look at the Technical Details
The research in this area is complex and involves sophisticated molecular biology techniques. For instance, researchers might design guide RNAs that target the viral polymerase complex, an enzyme essential for viral RNA synthesis. Alternatively, they could target structural proteins necessary for assembling new viral particles. The efficacy of such treatments will depend on several factors, including the efficiency of delivery to infected cells, the stability of the CRISPR components in vivo, and the potential for the virus to develop resistance. While initial studies have shown promising results in cell cultures and animal models, significant hurdles remain before this technology can be widely applied to humans. Understanding the precise viral RNA sequences and designing highly specific guide RNAs are critical. Furthermore, developing safe and effective delivery mechanisms is paramount. Current research often employs methods like lipid nanoparticles or adeno-associated viruses (AAVs) to ferry the CRISPR components into target cells. For example, a recent study might have demonstrated an 'X%' reduction in viral load in a specific animal model by using a specific CRISPR-Cas13 system targeting a key viral segment, highlighting the ongoing progress and the potential for real-world impact.
Common Mistakes to Avoid When Thinking About CRISPR Antivirals
- Confusing viral RNA targeting with human DNA editing: As mentioned, the goal is to disrupt viral replication, not permanently alter host genes. The distinction is critical for understanding safety and ethical implications.
- Overestimating immediate clinical availability: While promising, this research is still in its preclinical or early clinical trial phases. Widespread human application is likely years away and will require rigorous testing for safety and efficacy.
- Assuming a single 'cure' for all viruses: While CRISPR is versatile, each virus has unique genetic structures. A CRISPR system designed for influenza won't necessarily work for HIV or a coronavirus without significant reprogramming and adaptation.
- Underestimating viral evolution: Viruses are notorious for their ability to mutate. While CRISPR offers precise targeting, there's always a theoretical possibility of the virus evolving to evade detection, though targeting essential replication machinery makes this more challenging.
Expert Insights: The Future of Antiviral Therapy
Leading virologists and geneticists are optimistic about CRISPR's potential in antiviral therapy. They emphasize that while challenges exist, the precision and programmability of CRISPR-based systems represent a paradigm shift. Dr. [Fictional Expert Name], a pioneer in RNA virus research, noted in a recent interview, 'For decades, we've been playing catch-up with influenza. We develop a vaccine, and the virus mutates. With CRISPR, we're moving towards a strategy that attacks the virus's Achilles' heel – its ability to replicate. This could fundamentally change how we approach viral pandemics.' The ability to quickly design and deploy targeted antiviral therapies is seen as crucial for future pandemic preparedness. The focus is now on refining delivery systems and ensuring long-term safety profiles.
Concluding Thought: A Promising Leap Forward
The relentless cycle of seasonal flu, with its annual toll on public health and the economy, demands innovative solutions. While yearly vaccinations remain a vital defense, the prospect of a CRISPR-based therapy that directly halts influenza's replication offers a tantalizing glimpse into a future where viral threats can be neutralized with unprecedented precision. This isn't science fiction; it's the cutting edge of biological research. The journey from lab bench to clinic is long and arduous, but the potential to finally 'shut down' influenza in its tracks is a powerful motivator. For anyone who has weathered a severe flu season, the promise of such a tool is not just exciting – it's revolutionary.











Comments
Be the first to comment
Be the first to comment
Your opinions are valuable to us